Almost all common metals share several properties to some extent, like electrical conductivity. Not all of them conduct electricity equally. That’s why copper remains the universal choice for conduction.
What about aluminum? The metal has quietly but steadily become a go-to choice for power lines, aerospace parts, and consumer electronics. However, the question remains – is aluminum conductive?
This article looks deep into the material’s electrical properties. It explores practical benefits + concerns from the perspective of electrical conduction. You can even compare it with other key conductors.
To-The-Point Answer: Is Aluminum Conductive?
Yes. Aluminum (Al) and its alloys are excellent conductors of electricity. However, the metal has about 61% of the electrical conductivity of copper.
What Does ‘Conductive’ Mean?
Conductivity refers to a material’s ability to allow electrons to flow freely. It enables the transmission of electrical current (as well as heat). Most metals in the periodic table are good conductors.
It’s because the atomic structure within a metal allows electrons to move freely with less resistance. The better the conductivity, the more efficiently a conductive material can carry electric current.
Electrical Conductivity of Aluminum Alloys
Aluminum (Al) possesses a high electrical conductivity of 3.77 × 10⁷ S/m (Siemens per meter) at 20°C.
The higher the number, the better the material conducts electricity. And the numeric value indicates excellent conductivity. Of course, copper has the upper hand with a 5.96 × 10⁷ S/m conductivity.
Meanwhile, Aluminum (Al) possesses a low electrical resistivity of 2.65 × 10⁻⁸ Ω·m (Ohm-meters).
Resistivity is the inverse/opposite of conductivity. A lower resistivity means less resistance to current flow. The low resistivity confirms its efficiency in transmitting electricity with minimal energy loss.
Additionally, Aluminum (Al) holds a high thermal conductivity of 235 W/m·K (Watts per meter-Kelvin).
The value reveals how well aluminum can conduct heat. For reference, it’s 401 W/m·K with pure copper. Aluminum is also a strong thermal conductor, used in heat sinks, radiators, and LED housings.
Why Aluminum for Electricity Despite Lower Conductivity?
- Global Infrastructure: Aluminum lays the foundation of overhead power cables across the US, India, and Brazil. The low weight reduces structural load and installation costs.
- Cost Advantage: Aluminum alloys are significantly cheaper than copper ones. That’s why it’s suitable for industrial-scale applications where budget matters.
- Corrosion Resistance: Highly active aluminum forms a protective oxide layer. The stable cover keeps the interior intact against outdoor and marine environments.
- Thermal Conductivity: Aluminum also conducts heat well, thanks to the free electrons. It’s used in heat sinks, HVAC systems, and automotive radiators.
Aluminum: Good Conductor of Electricity (How Aluminum Conduct Electricity?)
- Atomic Structure
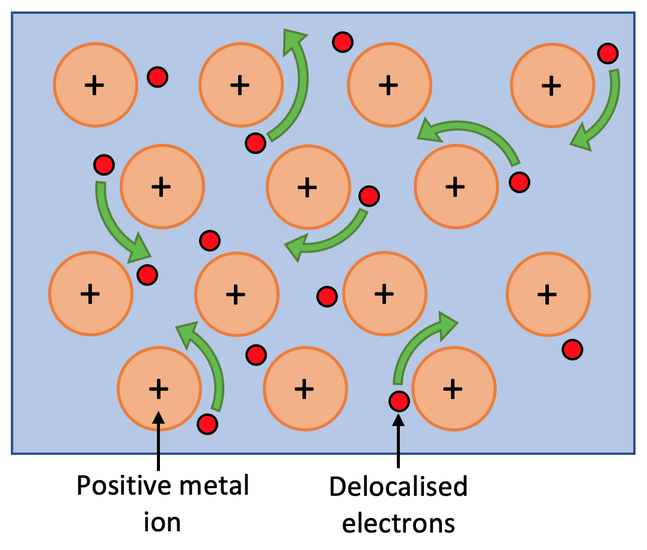
Aluminum’s ability to conduct electricity begins at the atomic level. Like other metals, aluminum atoms are arranged in a metallic lattice. There, valence electrons aren’t tightly bound to individual atoms.
Instead, they become delocalized to form a “sea of electrons” that can move freely within the metal.
Aluminum has three valence electrons in its outer shell. The three electrons are easily released into the electron sea, making aluminum such a good conductor.
The more free electrons a metal has, the better it can conduct electricity. Consider the electrons as riders or messengers. They carry electrical energy from one point to another with minimal resistance.
- Temperature Effects on Aluminum’s Conductivity
Aluminum’s conductivity is affected by temperature, like other metals. Atomic vibrations intensify at higher temperatures, which hinders the electron flow.
It leads to a slight increase in resistance, causing a somewhat lower electric conductivity. However, real-world applications can manage such changes.
Factors to Influence Aluminum’s Conductivity
- Alloy Composition
Pure aluminum (99.99%) holds the highest conductivity. It measures about 64.94% IACS (International Annealed Copper Standard).
Alloyed aluminum, such as 6061 or 6063, contains elements like magnesium, silicon, or zinc. They can improve strength for reduced conductivity.
For example, 6061-T6 aluminum has conductivity around 40% IACS only. It’s still usable but noticeably less efficient than pure aluminum.
Power grids mostly implement 1350-H19 aluminum, which is over 99.5% pure. Such an approach balances conductivity with mechanical strength.
- Temperature Sensitivity
As the temperature increases, the atoms vibrate more. It scatters the free electrons, which reduces conductivity. It marks a linear relationship in most cases: for every 1°C rise, conductivity drops slightly.
However, aluminum’s performance remains stable enough for applications across hot climates and high-load systems. It’s particularly true when the product gets designed with thermal expansion in mind.
In the humid and high-temperature South Asian regions, aluminum conductors are heavily used in rural electrification. The power lines remain effective and functional even in 35°C – 40°C environments.
- Oxidation Layer
Aluminum naturally forms a thin oxide layer (Al₂O₃) when exposed to air. The non-conductive layer is microscopically thin. Therefore, the oxide can barely intervene with the current flow.
Instead, the exterior layer protects the interior metal from exposure to corrosive agents. Such a built-in protection makes aluminum ideal for prolonged/extensive outdoor and marine uses.
Overhead transmission lines in coastal areas like Japan and Brazil rely on corrosion-resistant aluminum and its conductivity. The quality remains more or less the same, even with oxidation.
Al makes 90% of high-voltage transmission lines due to its low density, low cost, and response to the electric field. It gets preference over copper and other known metals for affordable electrification.
Aluminum Applications Based on Electrical Conductivity
- Power Transmission and Distribution
Aluminum has become the global standard for high-voltage power lines, and for good reason.
- Lightweight Advantage: Aluminum is about three times lighter than copper (2.70 g/cm³ vs. 8.96 g/cm³). It significantly reduces the structural load on towers and poles.
- Corrosion Resistance: The metal naturally forms an aluminum oxide layer. It seems ideal for withstanding outdoor and coastal environments.
- Cost Effectiveness: Aluminum is 30% – 50% cheaper than copper. Preferring the metal for large-scale infrastructure projects can save money.
ACSR Cables (Aluminum Conductor Steel Reinforced)
These cables combine aluminum strands for conductivity with a steel core for tensile strength. Such a combo enables long-distance transmission with minimal sag. They’re used in North America, India, and Brazil, where long spans with harsh climates demand strength and efficiency.

- Electrical Wiring and Components
Aluminum is also used extensively in low- and medium-voltage applications. The applications include –
- Building Wiring: Aluminum is used for branch circuits and service entrances in residential and commercial buildings.
- Bus Bars and Transformer Windings: Aluminum bus bars offer a lightweight, cost-effective alternative to copper in substations and switchgear.
- Connector Technology: Modern compression connectors, antioxidant pastes, and plated terminals have reduced the risk of oxidation-related resistance.
Real-World Example
Aluminum wiring is used in millions of American homes, built between the 1960s and 1970s. Today, updated standards and materials ensure safe and long-term performance.
- Electronics, Automotive, and Energy Systems
Aluminum’s dual conductivity, electrical and thermal, makes it indispensable in modern technology.
- Battery Casings and EV Power Systems: Aluminum is used in electric vehicle battery enclosures, bus bars, and inverters.
- Cooling Components: Its thermal conductivity (235 W/m·K) makes it ideal for heat sinks, radiators, and LED modules.
- Die-Cast Housings: Al-made housings provide EMI/RFI shielding in automotive ECUs, telecommunication devices, and industrial controls.
Real-World Examples
Tesla, BYD, and Volkswagen use aluminum extensively in EV platforms to reduce weight. Aluminum is found in mounting structures, inverters, and power electronics in renewable energy sectors.
Aluminum vs Copper: A Practical Conductivity Comparison
Better Electrical Conductor: Who Wins?
Copper remains the benchmark for electrical conductivity. It’s rated at 100% IACS (International Annealed Copper Standard) with a conductivity of 5.96 × 10⁷ S/m.
Aluminum has a conductivity of 3.77 × 10⁷ S/m, or 61% – 63% of an equivalent copper conductor. However, Al is 3x lighter, delivering sufficient conductivity efficiency as copper.
Aluminum requires a larger cross-sectional area to carry the same current as copper. It can affect connector size, space planning, and installation complexity.
Copper comes with a compact size and higher tensile strength. It seems ideal for tight spaces, high-load circuits, and precision wiring in electronic devices + buildings.
Dual Role: Electrical Conductor + Thermal Conductivity
Aluminum is one of the few metals to offer strong performance in electrical and thermal conductivity.
- Electrical conductivity: 3.77 × 10⁷ S/m, or 61% of copper’s conductivity.
- Thermal conductivity: 235 W/m·K, which enables efficient heat dissipation.
Such dual capability allows aluminum to serve multiple functions in a single component. It conducts electricity while simultaneously managing heat. It’s especially valuable in compact systems like electric vehicles, smartphones, and industrial control units.
- Heat Sinks and LED Housings
Aluminum is the standard material for heat sinks in CPUs, GPUs, and LEDs. It can absorb + dissipate heat quickly to ensure a longer lifespan and stable performance.
- Cooling Plates in Battery Modules
Aluminum cooling plates can regulate battery temperatures in EVs. It can further prevent thermal runaway to improve efficiency.
- Die-Cast Enclosures in Electronics
Housings from aluminum die casting provide EMI/RFI shielding, structural integrity, and heat dissipation. Al ensures signal protection + thermal stability in 5G base stations and IoT devices.
Limitations and Considerations
- Oxidation Layer: Protective but Problematic
The naturally formed oxide layer (Al₂O₃) due to air exposure is chemically stable. However, the non-conductive layer can increase contact resistance at electrical joints. It leads to potential heat buildup and subsequent failure.
Modern Solution: Surface treatments like electroless nickel plating, Alodine, and conductive pastes can break/bypass the oxide layer.
- Creep and Softening Under Heat and Pressure
Aluminum is more prone to creep. It’s a slow yet permanent deformation under sustained stress/heat. Therefore, Al connections can loosen over time, especially under thermal cycling or mechanical load.
Creep Risk Zones: Transformer terminals, bus bars, and switchgear joints where pressure + heat are almost constant.
- Connector Compatibility and Installation Challenges
Aluminum requires specialized connectors and installation practices for safe + efficient performance. Antioxidant compounds (Noalox or Penetrox) prevent oxidation for enhanced conductivity.
AL/CU-rated terminals accommodate aluminum and copper conductors. It reduces the risk of galvanic corrosion when dissimilar metals are joined.
- Not for Very High-Temperature Environments
Aluminum has a lower melting point than copper (660°C vs 1085°C). Even the thermal expansion is high, making it less suitable for extreme heat conditions.
The metal’s resistivity also increases more rapidly with temperature. Such changes can affect performance in high-current applications unless carefully engineered.
How to Enhance Aluminum Conductivity?
- Alloy Optimization (Aluminum Foil)
Pure aluminum lacks mechanical strength for demanding applications. Engineers introduce small amounts of magnesium (Mg) or silicon (Si) to create alloys like 6061 and 6101. They strike a balance between electrical performance and structural integrity.
- Surface Treatments
Mechanical brushing, abrasive pads, or chemical cleaning agents (phosphoric acid-based solutions) can remove surface oxidation. Conductive powder coating, like nickel plating, Alodine, or silver-based pastes, improves electrical contact and corrosion resistance.
- Composite Conductors (Other Metals)
ACSR (Aluminum Conductor Steel Reinforced): A combination of aluminum strands with a steel core for mechanical strength and long-span transmission.
Aluminum-Copper Hybrids: Copper-grade conductivity with aluminum’s weight advantage in high-load switchgear, EV bus bars, and smart grid nodes.
Frequently Asked Questions (FAQs)
- Is aluminum conductive enough for home wiring?
Yes. Aluminum has made it to residential wiring, especially for service entrances and branch circuits. Modern connectors and antioxidant compounds ensure safe performance.
- Does aluminum conduct electricity better than steel?
Absolutely. Aluminum’s conductivity is 61% of copper’s. Meanwhile, steel is rated 10% IACS only. That’s why Al remains far superior for electrical applications.
- Can aluminum be used in underground cables?
Yes. Aluminum is used in underground power distribution, especially in urban and rural grids. Its corrosion resistance and cost-efficiency seem ideal for buried installations.
- Is aluminum safe for high-voltage transmission?
Yes. Aluminum is the global standard for high-voltage lines. It comes from the metal’s lightness and ability to span long distances with minimal sag (ACSR cables).
- Does aluminum lose conductivity over time?
Not significantly. Oxidation may increase contact resistance, but the core conductivity remains stable. Proper maintenance and connectors mitigate long-term issues.
Conclusion
Aluminum, one of the most versatile metals, can conduct electricity to a great extent. It doesn’t match copper’s conductivity watt-for-watt for sure. Still, the advantageous balance of different properties, including electrical conductivity, remains unmatched.
Get Your Aluminum Products for Electrical Purposes from HONJENNY
Honjenny has been a top aluminum name with a careful understanding of what you need. We have been thriving through innovation, commitment, and precision for 30 years. Contact us to know further.