Aluminum (Al) is indeed a versatile and heavily used metal in modern manufacturing. Behind its universal usability lies a critical yet neglected thermal property – the melting point.
Understanding aluminum melting point is mandatory for precision in manufacturing processes. It’s because the temperature control directly impacts product quality and energy efficiency.
This article explores the exact melting point of aluminum. You can even examine its influencing factors and why this temperature plays such a crucial role in industrial applications worldwide.
What Is the Melting Point of Aluminum?
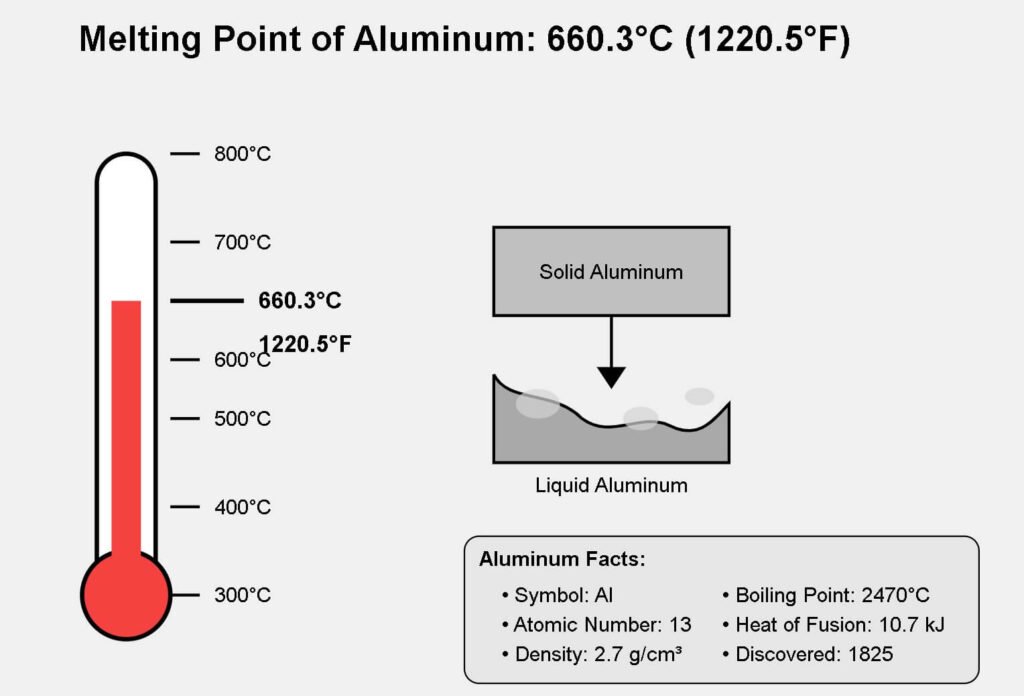
The melting point of aluminum is 660.3°C (1220.5°F) under standard atmospheric pressure.
The well-defined value specifically refers to pure aluminum, rated at 99.99% purity. However, aluminum alloys are the ones in industrial/commercial uses. The alloying elements can significantly alter the point.
What Does “Melting Point” Mean in Materials Science?
It indicates the temperature at which a solid changes into a liquid phase under normal atmospheric pressure. Any crystalline metal, like aluminum changes sharply at a defined temperature.
More specifically, it’s the point where interatomic bonds weaken enough to let atoms move freely. Such free movements turn the rigidity into a liquid state. It can impact a crucial physical property for –
- Die casting: Ensures precise mold filling without overheating.
- Welding: Guides heat input to avoid burn-through or weak joints.
- Extrusion: Helps maintain flow consistency during shaping.
- Recycling: Enables efficient melting and purification of scrap aluminum.
Aluminum Melting Point by Type and Alloy
Pure Aluminum (99.99%)
Exact Melting Point: 660.32°C (1220.58°F)
Pure aluminum transitions sharply at a single temperature due to its uniform atomic structure. It lacks a “mushy zone” that’s ideal for thermal modeling and casting equipment.
Common Aluminum Alloy Series
A lower melting point range in alloys often results from eutectic compositions. Alloying elements disrupt the aluminum lattice and reduce the energy needed for phase change.

Melting Point vs Solidification Range
Material melting (for alloys) doesn’t occur at a single temperature but across a range. The phenomenon is defined by –
- Solidus: Temperature at where melting begins.
- Liquidus: Temperature where the alloy is fully liquid.
- Mushy Zone: The intermediate phase where solid and liquid coexist.
Alloys with wide mushy zones (Al-Si) require careful thermal control to avoid defects. Narrow mushy zones allow faster solidification with superior dimensional accuracy.
Melting Aluminum: Die Casting Aluminum Alloys
Die casting alloys of aluminum enable melting at relatively low temperatures. Manufacturers can get rapid mold filling and reduced thermal stress. For instance,
- A380 (510°C – 595°C): Excellent castability, corrosion resistance.
- A383 (510°C – 593°C): Enhanced flow for thin-wall components.
- A360 (555°C – 596°C): Superior strength and pressure tightness.
Such lower melting ranges reduce the energy consumption in melting furnaces. Minimized oxidation and gas entrapment can improve mold life and surface finish.
Factors to Cause Different Melting Points
The temperature of 660.3°C (1220.5°F) is far from fixed in real-world applications. Aluminum alloys’ melting point varies significantly due to distinctive conditions.
- Alloy Composition
Aluminum is rarely used in its pure form across industrial applications. Instead, the metal is alloyed with elements like –
- Silicon (Al-Si): Improves castability and lowers the melting point.
- Magnesium (Al-Mg): Enhances strength and corrosion resistance.
- Copper (Al-Cu): Boosts hardness and thermal conductivity.
Such alloying exhibits a melting range of 480°C to 660°C, depending on the exact composition.
- Impurities + Aluminum Oxide
Trace elements like iron (Fe), silicon (Si), and zinc (Zn) remain present unintentionally. Those metals can disrupt uniform melting as –
- Iron: Intermetallic compounds resist melting, causing uneven solid phase transitions.
- Silicon: Even in small amounts, it lowers the melting point and alters solidification behavior.
- Fine grains: Promote uniform melting due to consistent atomic spacing.
- Coarse grains or stressed microstructures: Localized melting or delayed phase transitions.
- Environmental + Processing Conditions
Under high pressure, the melting point can increase slightly due to compression of atomic bonds. Conversely, reduced pressure (in vacuum environments) may lower the melting point marginally.
- Air melting: It comes with the risk of oxidation. The process forms a surface layer of aluminum oxide (Al₂O₃) that can interfere with melting.
- Vacuum melting: The process can prevent oxidation to ensure cleaner melts. It’s highly preferred for high-purity or aerospace-grade alloys.

Lower or Higher Melting Point: Aluminum vs Common Metals
Al’s melting point places it in the moderate range compared to other metals. It’s another reason for aluminum to be favored in energy-efficient production, rapid casting, and the recycling process.
Likewise, different alloys/grades (variations) within a metal or material are subjected to distinctive melting points.
Why is Aluminum Melting Point So Important?
The melting point of aluminum [660.3°C (1220.5°F) for pure aluminum] isn’t a simple technical detail. Instead, it decides on how the metal behaves, performs, and transforms across various industries.
a. Manufacturing for Industrial Applications
Aluminum’s relatively low melting point enables efficient casting into complex shapes. Molten Al is injected into steel molds under high pressure in die casting. The value itself directly affects –
- Mold design: Lower temperatures reduce thermal stress on molds, extending their lifespan.
- Alloy selection: Alloys like A380 are chosen for their melting behavior and flow characteristics.
b. Preventing Porosity, Shrinkage, and Oxidation
- Porosity: Overheating can trap gases, causing internal voids. Controlled melting minimizes gas solubility.
- Shrinkage: Uniform cooling prevents dimensional inconsistencies.
- Oxidation: Al oxidizes rapidly above its melting point. In inert or vacuum environments, oxidation is suppressed, improving part quality.
The German automotive sector, based on aluminum die casting, produces lightweight engine blocks with minimal porosity and high surface integrity.
c. Welding / Joining (Aluminum Extrusions)
The right temperature determines the optimal heat input to fuse aluminum without compromising its structure. Too much heat can cause warping or burn-through, while too little leads to weak joints.
- TIG and MIG welding techniques are calibrated to aluminum’s melting point for precision.
- Heat-affected zones (HAZ) must be carefully managed to preserve the mechanical strength.
Aluminum’s high thermal conductivity means heat disperses quickly during fusion welding. If temperatures exceed the melting point uncontrollably –
- Burn-through can occur, especially in thin sheets.
- Weak joints may form due to incomplete fusion or overheating.

d. Filler Metals with Lower Melting Points
To mitigate the risks associated with burn-through/weak joints, welders use filler rods (ER4045 or ER4043). They have slightly lower melting points than the base aluminum to ensure –
- Smooth fusion without overheating the parent metal.
- Reduced cracking in the heat-affected zone.
Aerospace manufacturers in the US use automated TIG welding systems with precise thermal control. It effortlessly joins aluminum fuselage panels, ensuring structural integrity under flight stress.
e. Material Recycling against Different Metals
Aluminum’s melting point plays a vital role in energy-efficient recycling. Melting scrap aluminum requires only 5% of the energy needed to produce primary aluminum from bauxite ore.
- Recovery Quality: Controlled melting ensures more impurities are separated, maintaining integrity.
- Global Scale: Over 95% of aluminum cans are recycled annually with efficient melting + reprocessing.
f. Thermal Conductivity
Aluminum’s high thermal conductivity (235 W/m·K) is closely tied to its melting point. As it approaches the melting point temperature (depending on the alloy) –
- Heat spreads rapidly, making it ideal for heat sinks in electronics.
- Uniform melting ensures consistent casting and extrusion results.
h. Ductile Behavior via Melting Point Depression
Ductility increases with the approach to the melting point. It allows aluminum to be drawn into wires or rolled into sheets. Even the atomic structure transforms to alter some mechanical properties.
There are other secondary important factors. Fatigue resistance feels more like other factors related to other elements. Your next project may require metals or other elements with low volatility.
Measuring and Controlling Aluminum Melting Temperature
Precise measurement and subsequent control of aluminum melting temperature are obligatory. Casting, welding, extrusion, and recycling – such intuitive measures are applicable across all industries.

How Is Aluminum’s Melting Temperature Measured?
- Thermocouples: They’re widely used in furnaces and crucibles. Direct insertion into molten aluminum provides real-time temperature readings. However, it must be protected from oxidation and corrosion.
- Infrared Pyrometers: The non-contact devices measure surface temperature using infrared radiation. Advanced models like AMETEK Land’s pyrometers offer ±1°C accuracy under industrial conditions.
- Thermal Profiling Systems: Such mechanisms are used in batch and continuous furnaces. Systems like Fluke’s Datapaq® Furnace Tracker monitor temperature across multiple zones for uniform heating.
Why Temperature Control Matters?
Overheating aluminum can degrade molds, increase oxidation, and cause porosity. Undermelting leads to incomplete mold filling and poor surface finish. Controlled melting ensures –
- Mold longevity.
- Dimensional accuracy.
- Reduced scrap rates.
Temperature-controlled die casting in German automotive foundries reduces defect rates by up to 30%, improving the production process.
- Aluminum’s thermal + high electrical conductivity demands tight control. Filler metals are selected with slightly lower melting points to ensure smooth fusion.
- Meanwhile, aluminum billets are preheated to 400°C – 500°C, below the melting point, to enhance ductility. Infrared sensors monitor surface temperature to prevent overheating and cracking.
- Aluminum frames across the Indian solar panel industry are extruded with temperature-controlled systems to impart flexibility and corrosion resistance.
Recycling aluminum saves 95% of the energy required for primary production. Its implementation can reduce CO₂ emissions by up to 9 tons per ton of aluminum recycled.
Global Standards
- ISO 8062 and ASTM B179 outline temperature control protocols for aluminum casting.
- Companies like WIKA and AMETEK Land provide high-precision instruments for aluminum temperature monitoring in over 50 countries.
Equipment and Furnace Types
Melting aluminum efficiently and safely requires selecting the right furnace and control systems based on scale, alloy type, and production goals.
- Induction Furnaces: Use electromagnetic fields to heat Al rapidly and uniformly. They’re ideal for precision melting and small-batch alloying. You can get more energy efficiency and clean operation.
- Reverberatory Furnaces: They’re common in large-scale foundries. Indirect heating of aluminum reflects the flame from the roof. The mechanism seems suitable for bulk melting and recycling operations.
- Crucible Furnaces: Ceramic or graphite crucibles initiate the heating process via gas/electricity. It’s the best for small-scale casting, prototyping, and artisanal work.
- Electric Resistance Furnaces: Heating takes place via resistive coils with precise temperature control. They’re exclusively used in laboratories and controlled alloy development.

Safety Protocols
Melting aluminum involves high temperatures and reactive materials. Strict safety measures are essential to prevent accidents.
- Moisture Hazards and Explosions: Water or moisture in scrap/tools can cause violent steam explosions when exposed to molten aluminum. All materials must be pre-dried before contact.
- Protective Equipment Requirements: Workers must wear heat-resistant gloves, face shields, flame-retardant clothing, and respirators (if fluxes or fumes are present).
- Handling Molten Metal (Al) Safely: Deploy ceramic-coated tools to avoid contamination. Always maintain clear pathways and spill containment zones.
- Emergency Procedures: Install thermal cutoff systems and gas leak detectors. Train the staff in burn treatment, spill response, and evacuation protocols.
Frequently Asked Questions (FAQs)
- Is aluminum toxic when melted?
Pure aluminum is non-toxic when melted. However, fumes from contaminated or painted aluminum can be hazardous. Therefore, proper ventilation is essential.
- Why is aluminum’s melting point important in 3D printing?
It determines the laser power and cooling rates in metal additive manufacturing. The decision directly affects layer adhesion and part strength.
- Can aluminum melt in a house fire?
Yes. House fires can reach 1000°C. Therefore, it can exceed aluminum’s melting point, causing structural aluminum elements to fail.
- Does aluminum melt in sunlight or extreme heat outdoors?
No. Even in desert conditions, ambient temperatures rarely exceed 60°C. It’s far below aluminum’s melting point.
- What happens to aluminum when it’s overheated?
The aluminum oxidizes rapidly, forming a brittle aluminum oxide layer. Such an occurrence can compromise welds and casting quality.
- Why is aluminum used in heat exchangers despite its melting point?
Its high thermal conductivity and corrosion resistance make it ideal. And the melting point is sufficient for most operating conditions.
Conclusion
The melting points of pure aluminum and aluminum alloys are more than scientific facts. This thermal threshold, when considered properly, empowers manufacturers to cast/weld with confidence. The melting point of aluminum lays the foundation of smarter, cleaner, and more efficient production.
Precise Aluminum Product Manufacturing at HONJENNY
Honjenny has been a household name in the aluminum manufacturing industry for 30 years. We excel in aluminum with innovation, commitment, and precision. Contact us to know more about the services.



